You can feel proud in the knowledge that your participation will be helping us to develop new treatments for future generations.
We aim to offer you a relaxed and friendly environment whenever you visit us at our modern, purpose-built unit, where our team of dedicated staff are looking forward to welcoming you.
With easy access via all major rail, tram and bus networks, you’ll find us situated within the Wythenshawe Hospital, South Manchester, where you’ll have access to free, secure parking in our private car park.
Accommodated in one of our three 12-bed wards, keep busy in our fully equipped Volunteer Lounge area, or relax in our garden area and make use of the summer house, or unwind in our designated ‘quiet’ area.
Whether you’re here for a short, long or overnight stay, you’ll find our unit welcoming and comfortable with a range of facilities at your disposal:
To make your stay as comfortable as possible, the unit has its own catering facilities with all meals prepared in-house, and you will have access to beverages and snacks at all other times (depending on study-specific restrictions).
During your time with us, if you have any questions about any of our facilities, our staff are happy to help.


You will receive a payment for taking part in our trials. An independent Ethics Committee determines the amount you are paid depending on the length of the trial, number of visits and the number of overnights involved.
The Volunteer Services team will advise you what the compensation for the trial will be prior to your consent visit. They will also let you know, in detail, exactly what will be involved, and all about the medication that’s being tested. All trials take a specific time to complete and you’ll be told the exact start and finish dates.
Payment is made within 2 weeks of completion of the trial by BACs payment straight into your bank account.
Please note, if you’re receiving any type of benefit, you should always check with your benefit provider if the payment you receive from us will affect the benefit you are receiving.
Also, please be aware that although the payment you receive from us is tax exempt, depending on your own personal circumstances, some or all of your payment may exceed your personal tax allowance. Please be reminded that tax declaration to HMRC is your responsibility.
For volunteers deemed ineligible, payment is calculated on a pro-rata basis, related to the study procedures completed by the volunteer
Take a look at our current studies, the payments and what each study involves. To see if you’re eligible, ring 0800 655 6553.

For our early phase I trials we are seeking healthy volunteers aged 18 to 65 with no current medical conditions or regular prescribed medications.
For our later phase trials we require both males and females aged 18 to 75 that have a diagnosis of Allergies, Asthma, Bronchiectasis, Chronic Cough, COPD (Chronic Obstructive Pulmonary Disease), CKD (Chronic Kidney Disease), Cystic Fibrosis, Hypertension, Nasal Polyps, Psoriasis/ Eczema/ Dermatitis, Thyroid

One of the main benefits of taking part is that you will be able to obtain expert medical care associated with the trial. As part of the screening process to enter any study you will receive a comprehensive medical check-up free of charge. Screening medicals can include procedures such as lung function blowing tests, ECG, allergy tests and general blood tests.


All clinical trials are voluntary. You always have the right to choose whether or not you will take part in a clinical trial and you have the right to leave a clinical trial at any time, for any reason.

Clinical trials are only a small part of the research that goes into developing a new treatment. In all, about 1,000 potential drugs are tested before just one reaches the point of being tested in people. Before testing in real people, a new medication will have been meticulously researched and rigorously tested over a period of many years.
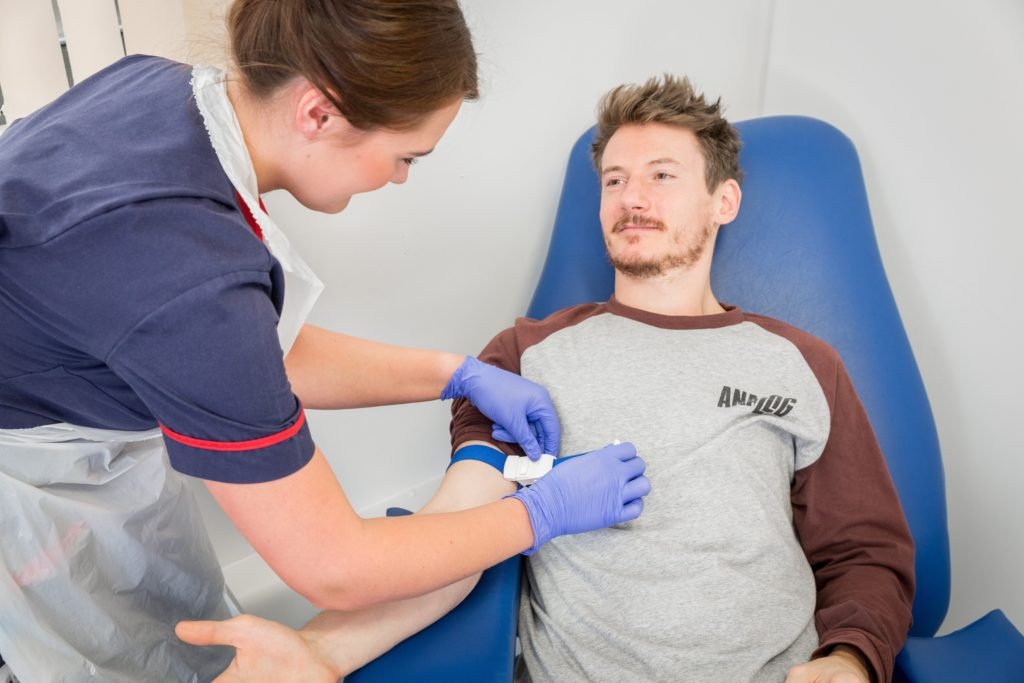
A new drug can cost more than £150 million to develop before reaching the market place. Clinical trials in people must go through several distinct phases before then can be considered for licensing.

The UK Clinical Research Collaboration (UKCRC) publishes a leaflet entitled ‘Understanding Clinical Trials’. This leaflet gives you more information about medical research and answers some questions you may have. A copy may be viewed online at www.ukcrc.org or may be obtained by writing to UKCRC, 20 Park Crescent, London, W1B 1AL
 Early phase I trials usually require healthy male volunteers in relatively small groups. The purpose of these trials is to assess the effects of the new medication on the body functions in a closely monitored environment.
Early phase I trials usually require healthy male volunteers in relatively small groups. The purpose of these trials is to assess the effects of the new medication on the body functions in a closely monitored environment.
 Once a new treatment has been assessed in Phase I trials and been found to be well tolerated, it can then go onto larger scale testing in patient volunteers. These later phase trials require volunteers who suffer from the illness which the trial drug is intended to treat. In these trials the new medication is assessed to see how well it performs in the treatment of the patients symptoms.
Once a new treatment has been assessed in Phase I trials and been found to be well tolerated, it can then go onto larger scale testing in patient volunteers. These later phase trials require volunteers who suffer from the illness which the trial drug is intended to treat. In these trials the new medication is assessed to see how well it performs in the treatment of the patients symptoms.
 Phase III clinical trials require a large number of patients, usually at least several hundred. These studies are generally conducted in many places across the country (or even across the world) at the same time. These trials compare the safety and effectiveness of the new treatment against the current standard treatment.
Phase III clinical trials require a large number of patients, usually at least several hundred. These studies are generally conducted in many places across the country (or even across the world) at the same time. These trials compare the safety and effectiveness of the new treatment against the current standard treatment.
 Phase IV studies look at drugs that have already been granted a license and have received approval therefore they are already available for doctors to give to patients but these studies are still needed to answer important questions.
Phase IV studies look at drugs that have already been granted a license and have received approval therefore they are already available for doctors to give to patients but these studies are still needed to answer important questions.